OR WAIT null SECS
FDA Extends PDUFA Date for B-VEC Gene Therapy for Dystrophic Epidermolysis Bullosa
An FDA announcement on this topical gene therapy for DEB pushes back the PDUFA date to May of this year, accompanying a regulatory update.
On January 5, the US Food and Drug Administration (FDA) announced that the PDUFA date for topical gene therapy B-VEC—designed to treat dystrophic epidermolysis bullosa (DEB)—would be extended to May 19 of 2023, along with a regulatory update.
The date change was announced by Krystal Biotech, Inc., in response to their submission of a manufacturing information update on the use of a replaced hardware unit in the manufacturing process.
“While we are disappointed that this change was viewed as a major amendment, we are committed to working with the FDA as it completes its review of the B-VEC application,” stated Krish Krishnan, CEO and Chairman of Krystal Biotech, Inc. “We will continue our commercial readiness efforts and upon approval bring this important treatment to DEB patients as soon as possible.”
According to the biotech firm’s officials, the unit did not alter their processing parameters or product contact materials, but nevertheless the update led to the FDA’s decision to review their application.
Krystal Biotech officials reported the prior completion of all pre-approval inspections of both clinical sites and their internal testing and manufacturing facilities.
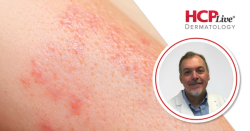
DEB—for which B-VEC is designed—is an uncommon genetic skin disorder that comes from COL7A1 genetic mutations and can lead to major skin and mucosal tissue fragility.
This is observed through DEB patients’ everyday wounds due to small touches.
B-VEC was designed by modifying a herpes simplex virus type 1 (HSV-1) vector.
Within the HSV-1 vector, 2 copies of the COL7A1 coding sequence are contained and the delivery system transcribes this viral DNA.
The DNA is translated into C7 which then results in its secretion within the extracellular space.
Recent phase 3 data on B-VEC therapy had strong results, indicating that about 70% of the wounds of DEB patients being assessed had closed by 6 months compared to only 20% given the placebo.